
Our stories
There’s a story to be told at every stage of the cancer journey – from prevention, to diagnosis, to treatment, to life with or beyond cancer. Here, you’ll find real experiences from Canadians across the country and learn how your support is helping the Canadian Cancer Society make a difference. Explore stories that highlight the impact of groundbreaking cancer research, support programs and services, advocacy, healthy living and more.
Our latest stories

4 research projects improving cancer care in rural and remote areas

Karen's Daffodil journey: Honouring her family through volunteering

Cassidy's story: Taking back control from cancer

Cancer and the workplace

10 ways to cope with cancer waiting periods

Joy and comfort at Camp Goodtimes

An inspiration at Relay For Life Youth

Training next-gen cancer researchers
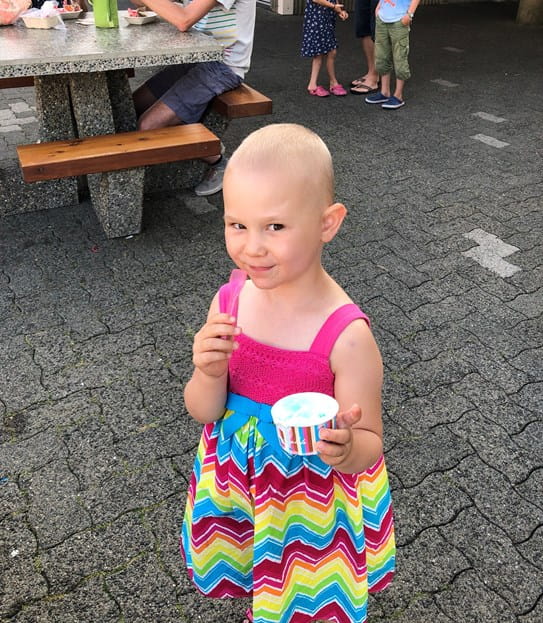
Paisley's childhood cancer story: Healing with joy as a family

The hidden costs of cancer

How advocacy creates an impact in cancer care
.jpg?rev=41204859c7fa49c4af27307b2e6d6cc9&cx=0.29&cy=0.44&cw=543&ch=623&hash=CB4AADBD1A77EAA5DD263799CBC8A5B8)
A drive to make others happy

Developing a new vaccine to treat triple-negative breast cancer

Finding hope with Dry Feb

Fund trailblazing cancer research

How to avoid caregiver burnout

Holiday comforts for cancer
.jpg?rev=8ae7f412322248758d68e0a4db6e2688&cx=0.5&cy=0.5&cw=543&ch=623&hash=E98C4D445AF531FB6BB20229A506FB34)
4 key cancer findings in 2023
